
The University Sports Centre (USC) has been renovated, and we would love to take you on a quick tour of the building. Whether you are a colleague at LBSP, a student, or an external sports enthusiast: everyone is welcome to…


The University Sports Centre (USC) has been renovated, and we would love to take you on a quick tour of the building. Whether you are a colleague at LBSP, a student, or an external sports enthusiast: everyone is welcome to…

Exactly one year after the first edition, the second national Skills Heroes Powered By final competition for Laboratory Technicians took place on 4th February 2026. Fifteen students from Intermediate Vocational Laboratory Education (MLO) competed at mboRijnland in Leiden for a…

The Academic Hospital Paramaribo (AZP) and the Leiden University Medical Center (LUMC) have been collaborating for approximately fifty years in the fields of neurosurgery and heart disease. Vascular surgeons Jan van Schaik and Daniël Eefting (also partly affiliated with Haaglanden…

The Leiden University Medical Center (LUMC), as a partner within the reNEW consortium, is once again receiving tens of millions for research into new treatments based on stem cells. The LUMC announced this. Novo Nordisk Foundation The partnership will receive…

Key Region Leiden has received a €100,000 grant from the Dutch Ministry of Economic Affairs to support entrepreneurs better in the Leiden region. The funding, that was awarded through the SME Services Action Agenda, will be used to strengthen connections…

Researchers have found that the risk of a brain tumour coming back can be better predicted by counting the number of immune cells in the tumour under a microscope. This result comes from research by the Leiden University Medical Center…

How can researchers tell whether a medicine will actually work? A new Leiden-based company called Omivera is developing a technology aimed at giving clearer answers and it has just received a seed investment from the investment fund UNIIQ to help…

LEIDEN, The Netherlands. February 04, 2026 – Leyden Laboratories B.V. (the “Company” or “Leyden Labs”) today announced landmark preclinical and clinical data in Science Translational Medicine supporting the development of their antibody-based nasal sprays to prevent influenza infection by neutralizing virus at…
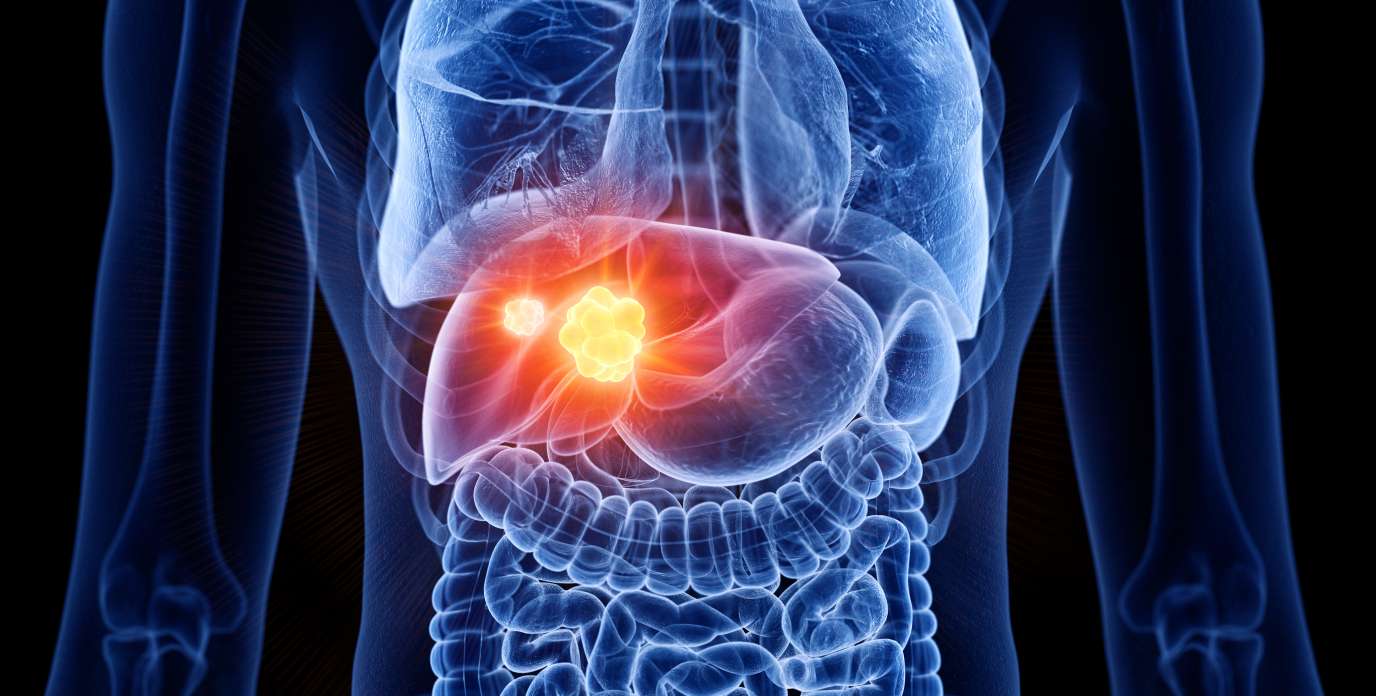
Levels Dx, a diagnostics company focused on improving early detection of liver cancer, announced the successful close of its latest investment round. The new funding will support a clinical validation study in 300 patients, a critical step in the development…
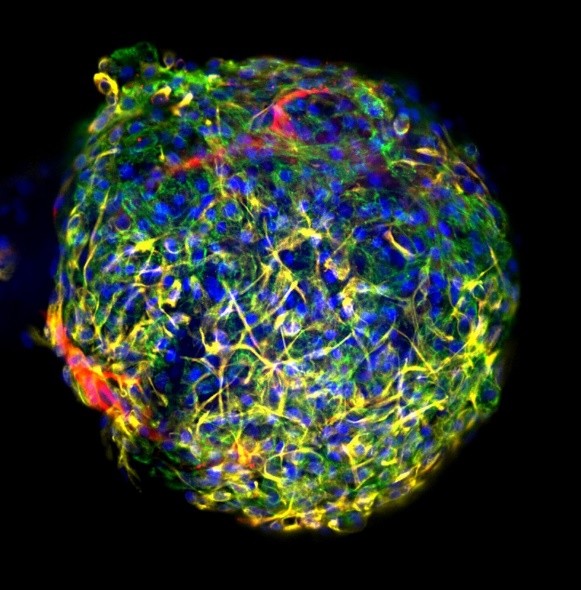
Researchers from Leiden University Medical Center (LUMC) and the Leiden-based biotech company Ncardia have joined forces to develop a new platform that enables the automated production of 3D cardiac microtissues. Using this platform, large numbers of drugs can be tested…

Dura Vermeer Commercieel Vastgoed and Cellares has signed a long-term lease for a new IDMO Smart Factory in building Nexus (9,741 sqm LFA) at Leiden Bio Science Park. The facility will serve as Cellares’ European headquarters and expand the company’sglobal…

The successful program for stimulating digitization in small and medium-sized businesses in the Leiden and Katwijk region is getting a sequel. Key Region Leiden, in cooperation with the province of South Holland, the participating regional municipalities and the regional entrepreneurs’…